

To the extent that the gas is ideal, the pressure depends linearly on temperature, and the extrapolation to zero pressure occurs at absolute zero. Consider a graph of pressure versus temperature made not far from standard conditions (well above absolute zero) for three different samples of any ideal gas (a, b, c). The constant volume gas thermometer plays a crucial role in understanding how could be discovered long before the advent of. Plots of pressure vs temperature for three different gas samples extrapolate to absolute zero.
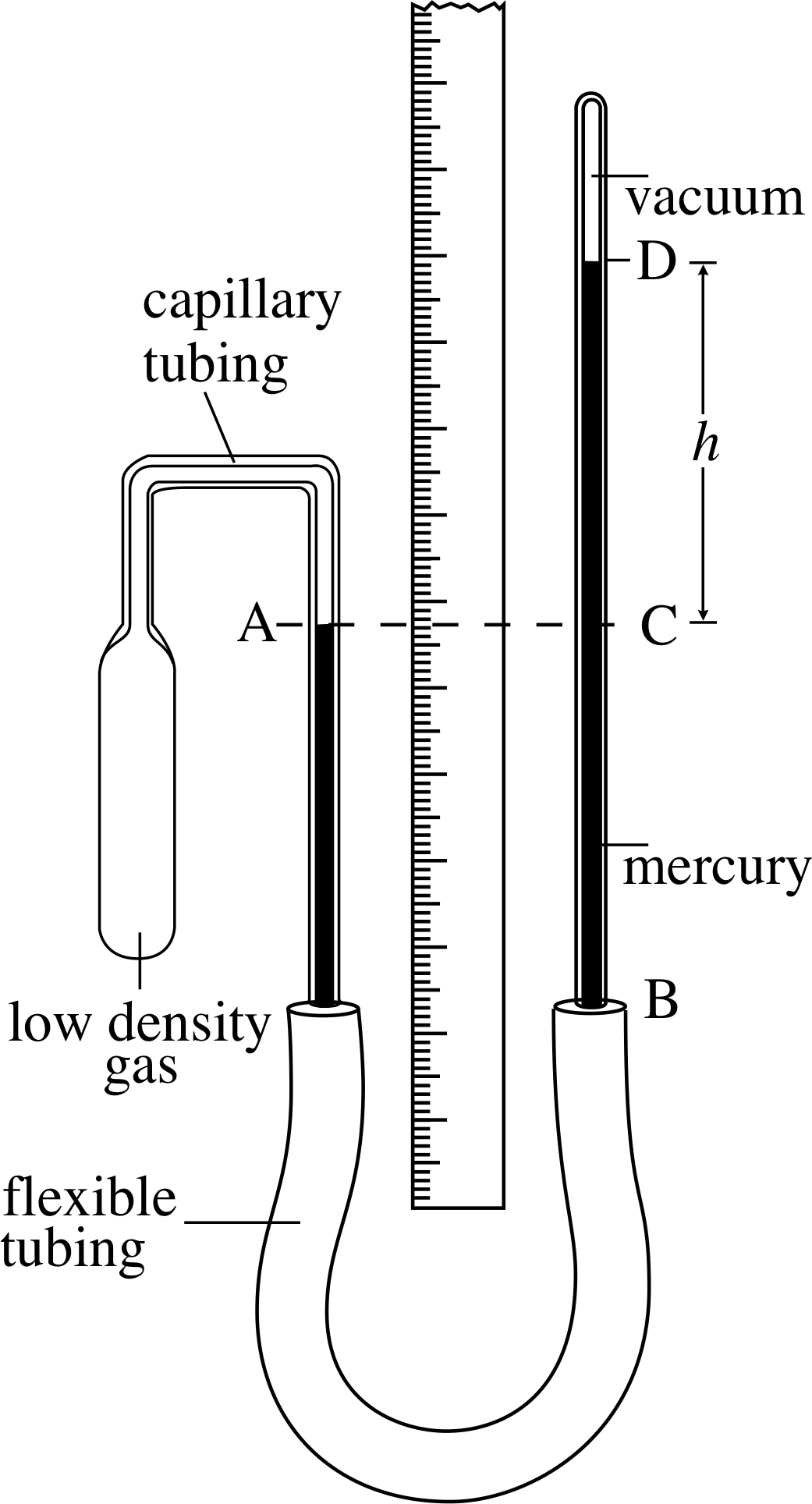
Explanation: The product of pressure and volume of a gas depends strongly on the temperature of. Constant volume gas thermometer In case of errors.


 0 kommentar(er)
0 kommentar(er)
